There were 6 million reactions in one year. Would you consider it safe?

VAERS (the U.S.

Numerous reviews of VAERS have found that only a tiny fraction of vaccine adverse events
A U.S. House Report similarly stated: “Former FDA Commissioner David A. Kessler has estimated that VAERS reports currently represent only a fraction of the serious adverse events.”31
Assuming VAERS captures 1 percent of adverse events (which is more than is estimated), then the number of adverse events reported to VAERS in 2016 would reflect for that year 5,911,700 adverse events, 43,200 deaths, 109,100 permanent disabilities, 413,200 hospitalizations, and 1,028,400 emergency room visits. If accurate, these figures are very troubling. Of course, these figures are merely estimates.
It would be far better if adverse
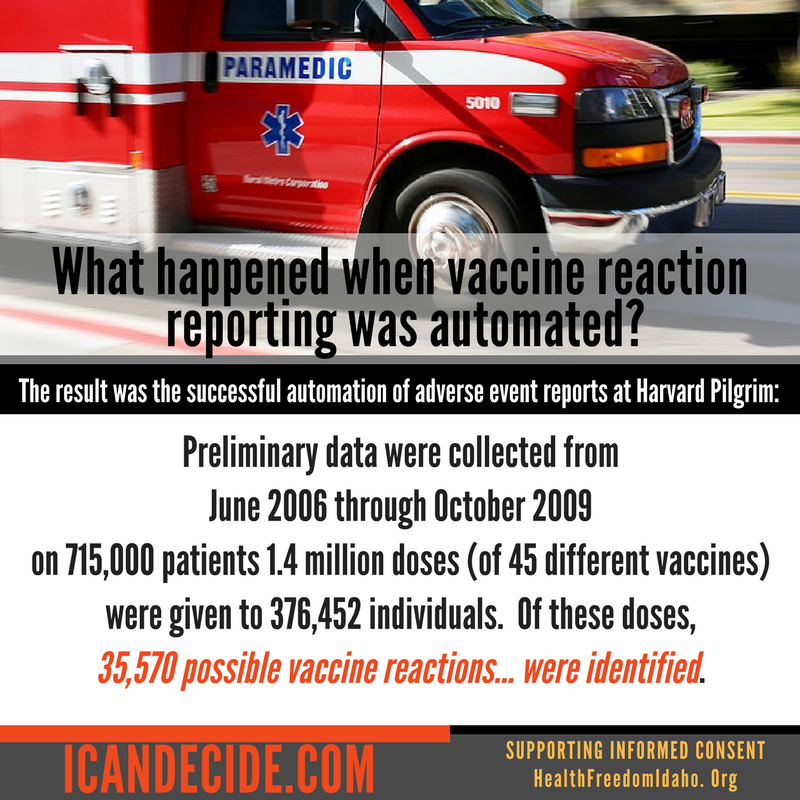
The Agency for Healthcare Research and Quality, an agency within HHS, sought to do exactly that in 2007 when it provided an approximately $1 million grant to automate VAERS reporting at Harvard Pilgrim Health Care.33 The result was the successful automation of adverse event reports at Harvard Pilgrim: Preliminary data were collected from June 2006 through October 2009 on 715,000 patients, and 1.4 million doses (of 45 different vaccines) were given to 376,452 individuals. Of these doses, 35,570 possible reactions
After automating adverse event reports at Harvard Pilgrim, the developers of this system asked the CDC to take the final step of linking VAERS with the Harvard Pilgrim system so that these reports could be automatically transmitted into VAERS. Instead, the CDC refused to cooperate.
As the Harvard grant recipients explained: Unfortunately, there was never an opportunity to perform system performance assessments because the necessary CDC contacts were no longer available and the CDC consultants responsible for receiving data were no longer responsive to our multiple requests to proceed with testing and evaluation.35
After three years and spending $1 million of taxpayers’ money, the CDC refused to even communicate with the HHS’ Harvard Medical School grant recipients. While HHS generally strongly supports automating public health surveillance systems, when it comes to vaccine safety, the CDC has only supported projects that would limit VAERS to passive surveillance
Automation would improve safety and address many of the long-standing issues and limitations raised by
Capturing “fewer than 1% of vaccine adverse events” thirty years after the passage of the 1986 Act is unacceptable – and potentially deadly.
Find out more at Vaccine Safety Paper fromIcandecide.com
- 32 https://healthit.ahrq.gov/ahrq-funded-projects/electronicsupport-public-health-vaccine-adverse-event-reporting-system
- 33 https://healthit.ahrq.gov/sites/default/files/docs/publication/ r18hs017045-
arus laz -fi nal-report-2011. pdf - 34 Ibid.
35 Ibid. - 36 http://www.ajpmonline.org/article/S0749-3797(12)00249- 8/pdf; https://www.ncbi.nlm.nih.gov/pubmed/26209838; https://www.
ncbi . nlm . nih . gov /pmc /articles/PMC4632204/ 37 I b id.






